An asymmetric hydrocyanation/Michael reaction of α-diazoacetates via Cu(i)/chiral guanidine catalysis†
Abstract
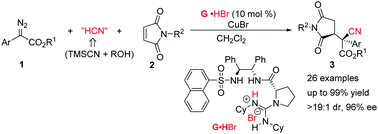
An asymmetric one-pot hydrocyanation/Michael reaction of α-aryl diazoacetates with trimethylsilyl cyanide, tert-butanol, and N-phenylmaleimides has been realized. Using a chiral guanidinium salt/CuBr catalyst, a series of cyanide-containing pyrrolidine-2,5-diones could be obtained in good yields with excellent diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...