Access to the most sterically crowded anilines via non-catalysed C–C coupling reactions†
Abstract
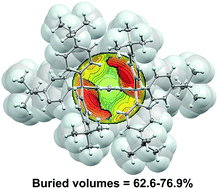
Variously substituted 2,6-bis(1,1-diarylethyl)anilines and 2,6-bis(trityl)anilines were prepared by a three-step high-yield process. Dimethyl-2-aminoisophtalate was modified by reaction with arylmagnesium bromides, and the hydroxy-derivatives obtained were etherified. Under the non-catalysed C–C coupling protocol, the formed bis[methyl(methoxy)diaryl]anilines react with various Grignard reagents to give highly substituted products. The buried volumes around the central nitrogen atom of the prepared compounds exceed the parameters for the known most sterically hindered anilines by about 20%.


 Please wait while we load your content...
Please wait while we load your content...