Synthesis of 3-(2-quinolyl) chromones from ynones and quinoline N-oxides via tandem reactions under transition metal- and additive-free conditions†
Abstract
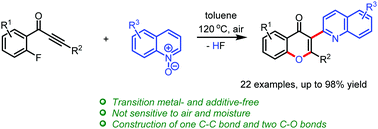
A novel method for the synthesis of 3-(2-quinolyl) chromones through a tandem [3+2] cycloaddition/ring-opening/O-arylation from ynones and quinoline N-oxides has been developed. This protocol proceeds under transition metal- and additive-free conditions and can be amplified to the gram level in 91% yield. 3-(1-Isoquinolyl) and 3-(2-pyridyl) chromones are also successfully synthesized using isoquinoline and pyridine N-oxides under basic conditions. Various heteroarene-contaning chromones were afforded in 30–98% yields, which are difficult to be obtained and are compounds of interest in pharmaceutical chemistry and chemical biology.


 Please wait while we load your content...
Please wait while we load your content...