Nickel-catalyzed regio- and diastereoselective hydroarylative and hydroalkenylative cyclization of 1,6-dienes†
Abstract
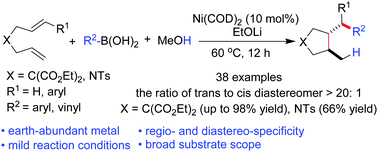
An unprecedented nickel-catalyzed hydroarylative and hydroalkenylative cyclization of unsymmetrically substituted 1,6-dienes with organoboronic acid was developed by using MeOH as the hydrogen source and employing commercially available Ni(η2-1,5-cyclooctadiene)2 as the catalyst. A series of biologically interesting cyclic products were afforded in moderate to excellent yields with high regio- and diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...