Acid promoted radical-chain difunctionalization of styrenes with stabilized radicals and (N,O)-nucleophiles†
Abstract
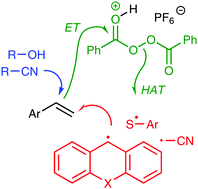
A difunctionalization of alkenes through sequential addition of a radical and a nucleophile has been developed, which is suggested to proceed by a radical chain mechanism not requiring a catalyst. An electron transfer step to the oxidant benzoyl peroxide is facilitated by protonation with a strong acid.


 Please wait while we load your content...
Please wait while we load your content...