Palladium-catalyzed aerobic synthesis of ortho-substituted phenols from cyclohexanones and primary alcohols†
Abstract
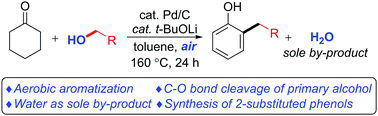
Due to the importance of phenols as structural cores and precursors of chemical products, synthesis of site-specific substituted phenols is highly desirable and a significant challenge. An aerobic palladium-catalyzed site-specific synthesis of ortho-substituted phenols from cyclohexanones and primary alcohols via an oxidation/aldol/dehydration/aromatization process has been developed. Various substituted cyclohexanones and primary alcohols are successfully transformed into ortho-substituted phenols. In addition, this catalytic reaction uses air as the terminal oxidant and generates water as the sole by-product. Furthermore, the method can also be extended to polyhydroxyl substituted substrates with high chemoselectivity between primary and secondary alcohols. This method provides a greener tool for synthesizing primary alkyl ortho-substituted phenols.


 Please wait while we load your content...
Please wait while we load your content...