Electrochemical synthesis of 3a-bromofuranoindolines and 3a-bromopyrroloindolines mediated by MgBr2†
Abstract
We report an efficient and environmentally friendly electrochemical approach to perform the bromo cyclization of tryptophol, tryptamine and tryptophan derivatives. The 3a-bromofuranoindolines and 3a-bromopyrroloindolines obtained are of interest in the total synthesis of natural products. This dearomative procedure relies on the generation of an electrophilic bromine reagent by the electrochemical oxidation of MgBr2. No organic byproducts are generated with this protocol which avoids the use of an additional electrolyte.
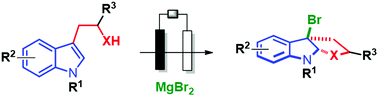


 Please wait while we load your content...
Please wait while we load your content...