Reversible oxidative-addition and reductive-elimination of thiophene from a titanium complex and its thermally-induced hydrodesulphurization chemistry†
Abstract
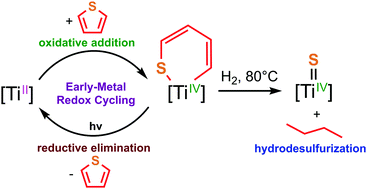
The masked Ti(II) synthon (Ketguan)(η6-ImDippN)Ti (1) oxidatively adds across thiophene to give ring-opened (Ketguan)(ImDippN)Ti[κ2-S(CH)3CH] (2). Complex 2 is photosensitive, and upon exposure to light, reductively eliminates thiophene to regenerate 1 – a rare example of early-metal mediated oxidative-addition/reductive-elimination chemistry. DFT calculations indicate strong titanium π-backdonation to the thiophene π*-orbitals leads to the observed thiophene ring opening across titanium, while a proposed photoinduced LMCT promotes the reverse thiophene elimination from 2. Finally, pressurizing solutions of 2 with H2 (150 psi) at 80 °C leads to the hydrodesulphurization of thiophene to give the Ti(IV) sulphide (Ketguan)(ImDippN)Ti(S) (3) and butane.


 Please wait while we load your content...
Please wait while we load your content...