A releasable disulfide-linked peptide tag facilitates the synthesis and purification of short peptides†
Abstract
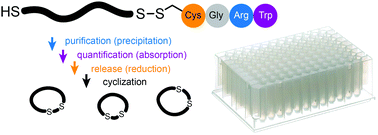
Combinatorial cyclization of hundreds to thousands of random linear peptides by structurally diverse chemical linkers offers access to large macrocyclic compound libraries. A bottleneck in the development of such libraries is the preparation of large numbers of short random linear peptides. Herein, we present a tag-based strategy that is not dependent on a throughput-limiting chromatographic purification step and thus enables parallel production of short peptides. In brief, peptides are synthesized on solid phase as conjugates with a disulfide-linked Cys-Gly-Arg-Trp tetra-peptide tag. The charged arginine residue in the tag allows for purification of the peptides by diethyl ether-precipitation and the tryptophan allows for quantification of the product by absorption measurement. Addition of a reducing agent releases the short peptides from the tag. The released sulfhydryl group in the peptide can readily be used for cyclization of the peptide library with bis-electrophilic linker reagents.


 Please wait while we load your content...
Please wait while we load your content...