Regioselective synthesis of 4-fluoro-1,5-disubstituted-1,2,3-triazoles from synthetic surrogates of α-fluoroalkynes†
Abstract
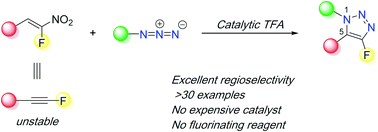
α-Fluoroalkynes are elusive molecules due to their instability and inaccessibility. Here, we show that α-fluoronitroalkenes can serve as synthetic surrogates of α-fluoroalkynes in [3+2] cycloaddition reactions with organic azides facilitated by a catalytic amount of trifluoroacetic acid (TFA). This work provides the first regioselective method to access 4-fluoro-1,5-disubstituted-1,2,3-triazoles.
- This article is part of the themed collection: Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...