Degrasyn exhibits antibiotic activity against multi-resistant Staphylococcus aureus by modifying several essential cysteines†
Abstract
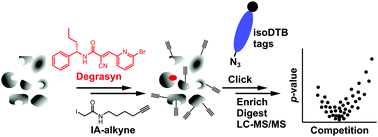
Degrasyn inhibits deubiquitination enzymes and has anti-cancer activity. We here show that it also exhibits antimicrobial activity against multi-resistant Staphylococcus aureus. Structure activity relationship studies demonstrate an important role of the electrophilic α-cyanoacrylamide moiety as a Michael acceptor. A suite of chemical proteomic techniques unraveled binding of this moiety to various cysteine residues of essential proteins in a reversibly covalent manner.


 Please wait while we load your content...
Please wait while we load your content...