Organocatalytic enantioselective synthesis of 2,5,5-trisubstituted piperidines bearing a quaternary stereocenter. Vinyl sulfonamide as a new amine protecting group†
Abstract
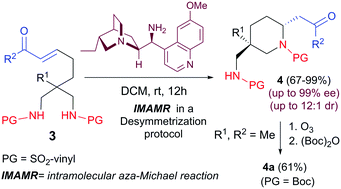
An organocatalytic desymmetrizing intramolecular aza-Michael reaction with vinyl sulfonamides as nucleophilic nitrogen source has been devised for the synthesis of a new family of 2,5,5-trisubstituted piperidines bearing a quaternary sterocenter. The process takes place with excellent levels of enantioselectivity and moderate to good diastereoselectivity. The vinyl sulfonamide moiety can be removed by means of an ozonolysis reaction.


 Please wait while we load your content...
Please wait while we load your content...