Methylation of geometrically constrained lysine analogues by histone lysine methyltransferases†
Abstract
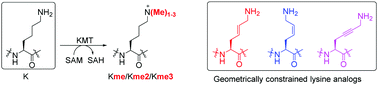
We report synthesis and enzymatic assays on human histone lysine methyltransferase catalysed methylation of histones that possess lysine and its geometrically constrained analogues containing rigid (E)-alkene (KE), (Z)-alkene (KZ) and alkyne (Kyne) moieties. Methyltransferases G9a and GLP do have a capacity to catalyse methylation in the order K ≫ KE > KZ ∼ Kyne, whereas monomethyltransferase SETD8 catalyses only methylation of K and KE.


 Please wait while we load your content...
Please wait while we load your content...