Conformationally rigid pyrazoloquinazoline α-amino acids: one- and two-photon induced fluorescence†
Abstract
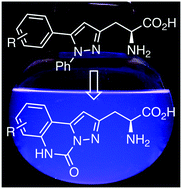
The synthesis and photophysical properties of a new class of α-amino acid bearing a rigid pyrazoloquinazoline chromophore are described. Confromational constraint of the amino acid side-chains resulted in high emission quantum yields, while the demonstration of two-photon-induced fluorescence via near-IR excitation signifies their potential for sensitive bioimaging applications.


 Please wait while we load your content...
Please wait while we load your content...