Highly regioselective ring-opening of epoxides with amines: a metal- and solvent-free protocol for the synthesis of β-amino alcohols†
Abstract
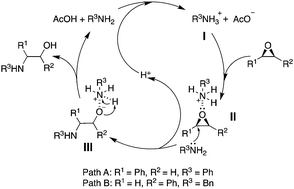
We herein report a metal- and solvent-free acetic acid-mediated ring-opening reaction of epoxides with amines. This process provides β-amino alcohols in high yields with excellent regioselectivity. Importantly, this epoxide ring-opening protocol can be used for the introduction of amines in natural products during late-stage transformations.
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...