The direct trifluoromethylsilylation and cyanosilylation of aldehydes via an electrochemically induced intramolecular pathway†
Abstract
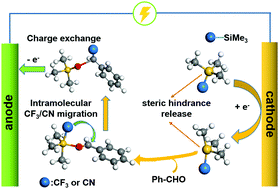
The initiator-free electrochemical trifluoromethylsilylation and cyanosilylation of aldehydes were developed in an undivided cell. A DFT study reveals that the direct cathodic activation of trimethylsilyl reagents significantly released the congestion around the ‘Si’ atom, allowing the Si–O bond affinity to form concerted anion intermediates with aldehydes. Thus, intramolecular –CF3 and –CN migration make the reactions much easier to carry out without initiators.


 Please wait while we load your content...
Please wait while we load your content...