Divergent C–H activation synthesis of chalcones, quinolones and indoles†
Abstract
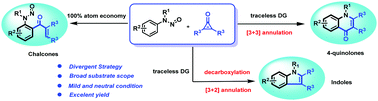
We here report a condition-controlled divergent synthesis strategy of chalcones, quinolones and indoles, which was achieved via a C–H activation reaction of N-nitrosoanilines and cyclopropenones. Variations of Ag salts are observed to be crucial for divergently constructing the three distinct chemical scaffolds. A Rh(I)- and Rh(III)-cocatalyzed decarbonylation/C–H activation/[3+2] annulation cascade reaction was developed for the synthesis of indoles. These methodologies are characterized by mild reaction conditions, high functional group tolerance, and amenability to gram-scale synthesis, providing a reference for future derivation of new chemical scaffolds by C–H activation.


 Please wait while we load your content...
Please wait while we load your content...