Photochemical fluoro-amino etherification reactions of aryldiazoacetates with NFSI under stoichiometric conditions†
Abstract
Herein, we report on a photochemical three-component reaction of aryldiazoacetates with NFSI and cyclic ethers. This method allows the introduction of both fluorine and a short ether sidechain using 1,4-dioxane as solvent in high yields via an ylide pathway as demonstrated by DFT calculations (26 examples, up to 99% yield).
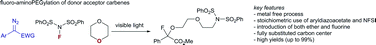


 Please wait while we load your content...
Please wait while we load your content...