Iridium-catalyzed B–H insertion of sulfoxonium ylides and borane adducts: a versatile platform to α-boryl carbonyls†
Abstract
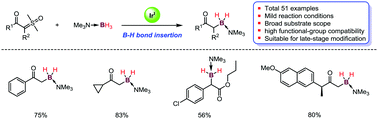
Iridium-catalyzed boron–hydrogen bond insertion reactions of trimethylamine-borane and sulfoxonium ylides have been demonstrated, furnishing α-boryl ketones in moderate to excellent yields in most cases (51 examples; up to 84%). This practical and scalable insertion reaction showed broad substrate scope, high functional-group compatibility and could be applied in late-stage modification of structurally complex drug compounds. Further synthetic applications were also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...