Electrochemical diselenylation of indolizines via intermolecular C–Se formation with 2-methylpyridines, α-bromoketones and diselenides†
Abstract
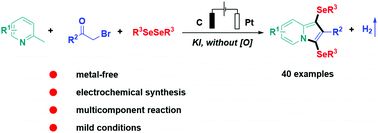
An efficient electrochemical system for the construction of diselenytlated indolizines from available pyridines, ketones and diselenides under undivided electrolytic conditions was developed. No external oxidants and transition-metal catalysts are needed for achieving this three-component tandem reaction realizing C–C, C–N and C–Se bond formations.


 Please wait while we load your content...
Please wait while we load your content...