Electrochemically induced crystallization of amorphous materials in molten MgCl2: boron nitride and hard carbon†
Abstract
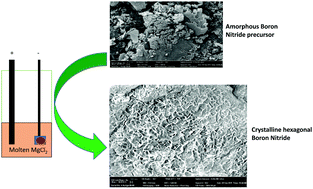
A novel and versatile strategy for the amorphous-to-crystalline transformation of boron nitride (BN) with the capability to control the degree of crystallization was developed through an electrochemical pathway using MgCl2 at low temperature (750 °C). This procedure can be extended to the transformation of amorphous carbon to graphite, which significantly reduces the energy and cost, accelerates the synthesis process and could potentially replace industrial graphite synthesis globally. Thus, the synthesized graphite exhibits much enhanced electrochemical performance at high charge–discharge rates (5C) compared to commercial synthetic graphite.


 Please wait while we load your content...
Please wait while we load your content...