Monomer-targeting affinity peptide inhibitors of amyloid with no self-fibrillation and low cytotoxicity†
Abstract
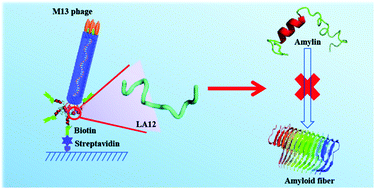
We utilized solution-phase biopanning to obtain a de novo peptide (LA12) that specifically bound to the core region of the human amylin monomer. LA12 stabilized the random coil conformation of amylin to suppress aggregation in a dose-dependent manner with the highest suppression effect of 78% and reduced the cytotoxicity of amylin.


 Please wait while we load your content...
Please wait while we load your content...