Transition metal-free cyclobutene rearrangement in fused naphthalen-1-ones: controlled access to functionalized quinones†‡
Abstract
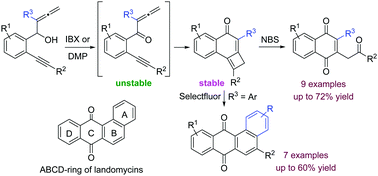
The controlled synthesis of 1,4-naphthoquinones and tetraphene-7,12-diones, which bear the ABCD-ring of landomycins, has been accomplished directly through oxidative rearrangement of common stable precursors, namely, previously non-isolable cyclobuta[a]naphthalen-4(2H)-ones.


 Please wait while we load your content...
Please wait while we load your content...