Sodium anilinide–cyclohexylamide pair: synthesis, characterization, and hydrogen storage properties†
Abstract
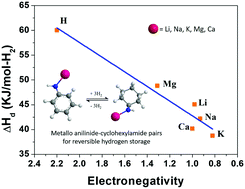
The lack of efficient hydrogen storage material is one of the bottlenecks for the large-scale implementation of hydrogen energy. Here, a series of new hydrogen storage materials, i.e., anilinide–cyclohexylamide pairs, are proposed via the metallation of an aniline–cyclohexylamine pair. DFT calculations show that the enthalpy change of hydrogen desorption (ΔHd) can be significantly tuned from 60.0 kJ per mol-H2 for the pristine aniline–cyclohexylamine pair to 42.2 kJ per mol-H2 for sodium anilinide–cyclohexylamide and 38.7 kJ per mol-H2 for potassium anilinide–cyclohexylamide, where an interesting correlation between the electronegativity of the metal and the ΔHd was observed. Experimentally, the sodium anilinide–cyclohexylamide pair was successfully synthesised with a theoretical hydrogen capacity of 4.9 wt%, and the hydrogenation and dehydrogenation cycle can be achieved at a relatively low temperature of 150 °C in the presence of commercial catalysts, in clear contrast to the pristine aniline–cyclohexylamine pair which undergoes dehydrogenation at elevated temperatures.


 Please wait while we load your content...
Please wait while we load your content...