Stereoselective synthesis of trifluoromethyl-substituted 2H-furan-amines from enaminones†
Abstract
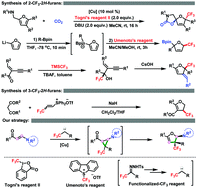
A straightforward strategy for synthesis of highly functionalized trifluoromethyl 2H-furans is described. The copper catalyzed method relies on a cascade cyclic reaction between enaminones and N-tosylhydrazones. This method allows the synthesis of 2-amino-3-trifluoromethyl-substituted 2H-furan derivatives carrying a quaternary stereogenic center as single diastereomers. The proposed reaction mechanism involves an amino-cyclopropane intermediate formed in the cyclopropanation of enaminones. The developed method tolerates a broad spectrum of functionalities, and the obtained 2H-furan derivatives are useful synthetic intermediates for preparing other trifluoromethyl-substituted compounds.


 Please wait while we load your content...
Please wait while we load your content...