The dual-catalyzed boryldifluoroallylation of alkynes: an efficient method for the synthesis of skipped gem-difluorodienes†
Abstract
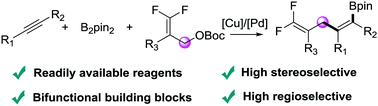
The Cu/Pd-catalyzed boryldifluoroallylation of alkynes was achieved, providing the corresponding skipped gem-difluorodiene scaffolds with high regio- and stereoselectivity in moderate to excellent yields. This new approach has good functional group compatibility for both alkynes and 3,3-difluoro-substituted allylic esters. Moreover, an array of synthetic building blocks, skipped dienes, trienes, and drug mimics can be obtained via further transformations.


 Please wait while we load your content...
Please wait while we load your content...