A rechargeable aqueous aluminum–sulfur battery through acid activation in water-in-salt electrolyte†
Abstract
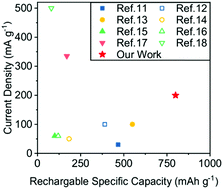
We demonstrated a rechargeable aqueous Al–S battery based on a water-in-salt electrolyte with the configuration Al‖Al(OTF)3 + LiTFSI + HCl‖S/C. The superconcentrated LiTFSI trapped water molecules to inhibit the hydrolysis of aluminum polysulfides in the cathode, and the HCl additive provided a mild acidic environment to enable repeatable oxidation–reduction reactions in the anode.


 Please wait while we load your content...
Please wait while we load your content...