Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: a synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity†
Abstract
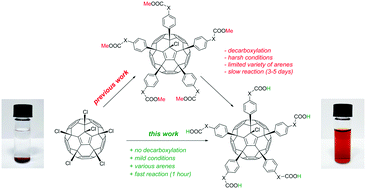
We report unprecedented Friedel–Crafts arylation of chlorofullerenes C60Cl6 and C70Cl8 with unprotected carboxylic acids as an efficient single-step synthesis of the inherently stable water-soluble fullerene derivatives. Using this method, a series of previously unaccessible compounds was obtained without chromatographic purification in almost quantitative yields. Promising anti-HIV activity comparable to characteristics of commercial drugs was demonstrated for some of these compounds.


 Please wait while we load your content...
Please wait while we load your content...