Unusual electrochemical N2 reduction activity in an earth-abundant iron catalyst via phosphorous modulation†
Abstract
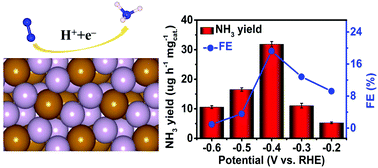
Fe-enabled high-performance ambient electrochemical N2 reduction still remains a big challenge. Here, we report the unusual role of phosphorous in modulating the electrochemical N2 reduction activity of an Fe catalyst. An FeP2 nanoparticle–reduced graphene oxide hybrid (FeP2–rGO) attains a large NH3 yield of 35.26 μg h−1 mgcat.−1 (7.06 μg h−1 cm−2) and a high faradaic efficiency of 21.99% at −0.40 V vs. reversible hydrogen electrode in 0.5 M LiClO4, outperforming the FeP–rGO hybrid (17.13 μg h−1 mgcat.−1; 8.57%). Theoretical calculations reveal that FeP2 possesses decreased catalytic activity for the hydrogen evolution reaction, higher N2 adsorption energy, and a larger number of active sites than FeP.


 Please wait while we load your content...
Please wait while we load your content...