Mass spectrometry reveals the assembly pathway of encapsulated ferritins and highlights a dynamic ferroxidase interface†
Abstract
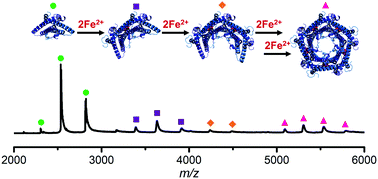
Encapsulated ferritins (EncFtn) are a recently characterised member of the ferritin superfamily. EncFtn proteins are sequestered within encapsulin nanocompartments and form a unique biological iron storage system. Here, we use native mass spectrometry and hydrogen–deuterium exchange mass spectrometry to elucidate the metal-mediated assembly pathway of EncFtn.


 Please wait while we load your content...
Please wait while we load your content...