Total synthesis of the Helicobacter pylori serotype O2 O-antigen α-(1 → 2)- and α-(1 → 3)-linked oligoglucosides†
Abstract
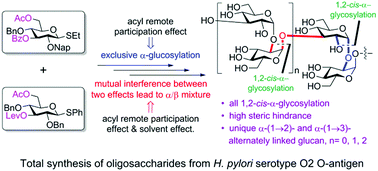
Exploiting synergistic remote participation effects of acyl groups at the O3 and O6 positions was key to the complete α-selectivity during the total synthesis of the unique (1 → 2)- and (1 → 3)-linked α-oligoglucosides from the Helicobacter pylori O2 O-antigen. Acyl remote participation and solvent effects were found to counteract during α-stereoselective glucosylations for the first time. The resulting antigen is a lead for the development of a carbohydrate-conjugate vaccine.


 Please wait while we load your content...
Please wait while we load your content...