Asymmetric construction of quaternary α-nitro amides by palladium-catalyzed C(sp3)–H arylation†
Abstract
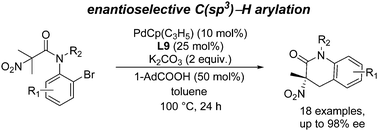
Pd-Catalyzed enantioselective C(sp3)–H arylation of N-(o-Br-aryl) anilides has been disclosed, and quaternary α-nitro amides were constructed with up to 98% ee. The presence of the nitro group on the substrate enables the progress of the reaction and the ready transformation of the product to optically active quaternary amino acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...