Regio- and chemoselective synthesis of nitrogen-containing heterocycles via the oxidative cascade cyclization of unactivated 1,n-enynes†
Abstract
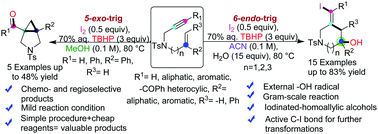
The development of a method for performing radical initiated intramolecular cascade cyclization of 1,n-enynes provided a competent protocol for producing structurally diverse complex heterocycles. Herein, we demonstrate I2/TBHP promoted, solvent controlled, regio- and chemoselective 5-exo-trig or 6-endo-trig radical cyclization of electronically unbiased 1,n-enynes to synthesize nitrogen-containing heterocycles. These metal-free protocols confer access to synthetically robust piperidine motif-bearing iodinated-homoallylic alcohol and pyrrolidine fused cyclopropane rings. The key factor in these methods is operational simplicity, and the environmentally friendly reaction system works under exceedingly mild conditions. Furthermore, the source of hydroxyl groups in the tertiary alcohols is indirectly confirmed with H218O labeled studies. Based on mechanistic studies, all of these cyclizations were initiated through single electron oxidation. The products with active C–I bonds present opportunities for further transformations.


 Please wait while we load your content...
Please wait while we load your content...