Au(i)/Au(iii)-Catalyzed C–N coupling†
Abstract
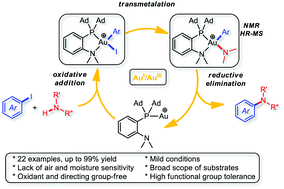
Cycling between Au(I) and Au(III) is challenging, so gold-catalyzed cross-couplings are rare. The (MeDalphos)AuCl complex, which we showed was prone to undergo oxidative addition, is reported here to efficiently catalyze the C–N coupling of aryl iodides and amines. The transformation does not require an external oxidant or a directing group. It is robust and works with a wide scope of aryl iodides and N-nucleophiles under mild conditions. Mechanistic studies, including the NMR and MS characterization of a key aryl amido Au(III) complex, strongly support a 2e redox cycle in which oxidative addition precedes transmetalation and reductive elimination is the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...