Stable, yet “naked”, azo radical anion ArNNAr− and dianion ArNNAr2− (Ar = 4-CN-2,6-iPr2-C6H2) with selective CO2 activation†
Abstract
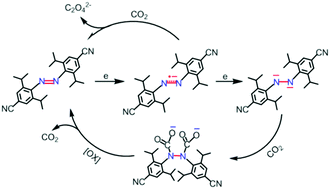
Azo radical anion 1˙− and dianion 12− have been isolated by one- and two-electron reduction of the azo compound 1 (ArNNAr, Ar = 4-CN-2,6-iPr2-C6H2) with alkali metals, respectively. The reduced species have been characterized by single-crystal X-ray analysis, EPR, UV and FT-IR spectroscopy, as well as SQUID measurements. The filling of one and two electrons in the π* orbital of the N–N double bond of 1 leads to a half-double N–N bond in 1˙− and a single N–N bond in 12−. The uncoordinated nature of these reduced species enables them to activate CO2. The exposure of 1˙− solution to CO2 led to the formation of oxalate anion C2O42−, while that of 12− solution to CO2 afforded the hydrazine dicarboxylate dianion [1-2CO2]2−, which reversibly dissociated back to 1 and CO2 upon oxidation.


 Please wait while we load your content...
Please wait while we load your content...