A competitive and highly selective 7-, 6- and 5-annulation with 1,3-migration through C–H and N–H – alkyne coupling†
Abstract
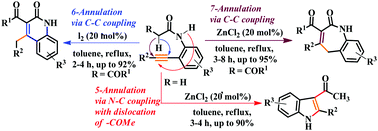
We demonstrated a highly competitive and selective C–C and N–C cross-coupled 7-, 6- and 5-annulation utilizing 2-ethynylanilides to afford functionalized 1H-benzo[b]azepin-2(5H)-ones, 2-quinolinones, and 3-acylindoles in high yield. ZnCl2 was found to be the smart catalyst for 7- and 5-annulation with 1,3-migration through C–H and N–H functionalization, respectively, whereas molecular iodine performed the C–H functionalized 6-annulation with a nonconventional 1,3 H-shift. The mechanism was investigated by intermediate trapping, control, and labeling experiments.


 Please wait while we load your content...
Please wait while we load your content...