Ba(B2OF3(OH)2)2 with well-ordered OH/F anions and a unique B2OF3(OH)2 dimer†
Abstract
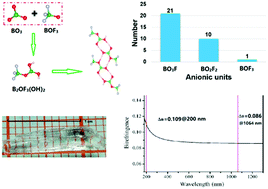
In minerals and artificial crystals, the OH and F groups often coexist and have a high probability of disordered structure. The OH/F position cannot be determined accurately in the crystal structure only by the direct X-ray diffraction method because of the poor contrast between O and F and the weak diffractivity of hydrogen atoms. In this work, a new fluorooxoborate Ba[B2OF3(OH)2]2, with a size of up to 12 × 4 × 3 mm3, has been synthesized successfully by a facile hydrothermal reaction. The BOF3 units with well-ordered OH/F positions are observed for the first time in alkali/alkaline earth metal fluorooxoborates. Owing to the selective fluorination of BO4, Ba(B2OF3(OH)2)2 exhibits a large birefringence and hence can be used as a DUV birefringent material. This work will guide the study of the structural chemistry of oxyfluorides.


 Please wait while we load your content...
Please wait while we load your content...