Met80 and Tyr67 affect the chemical unfolding of yeast cytochrome c: comparing the solution vs. immobilized state
Abstract
Urea-induced denaturation of the Met80Ala and Met80Ala/Tyr67Ala variants of S. cerevisiae iso-1 cytochrome c (ycc) was studied through variable temperature diffusive cyclic voltammetry and electronic absorption, CD and MCD spectroscopies. The susceptibility to unfolding of both variants – represented by the free energy of unfolding at denaturant infinite dilution, 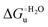 – is greater compared to the species showing an intact Met/His coordination, as observed previously for the same species immobilized onto a functionalized electrode. This is consistent with the role of the axial Fe–(S)Met bond and the H-bond network involving Tyr67 in stabilizing the polypeptide matrix in the heme crevice. Notably, we find that the unfolding propensity and axial heme iron coordination of the present Fe–(S)Met bond-deprived variants are affected by the motional regime of the protein. In particular, electrostatic adsorption onto a negatively charged SAM surface – which would mimic the phospholipidic inner mitochondrial membrane – facilitates unfolding compared to the solution state, especially at room temperature. This finding has physiological relevance related to the cytochrome c interaction with cardiolipin at the IMM in the early stages of apoptosis. Moreover, while both immobilized variants maintain the His/OH− axial heme iron coordination up to 7 M urea, the same species in solution are subjected to urea-induced replacement of the axial hydroxide ligand by a His ligand. The contributions of the enthalpic and entropic terms to
– is greater compared to the species showing an intact Met/His coordination, as observed previously for the same species immobilized onto a functionalized electrode. This is consistent with the role of the axial Fe–(S)Met bond and the H-bond network involving Tyr67 in stabilizing the polypeptide matrix in the heme crevice. Notably, we find that the unfolding propensity and axial heme iron coordination of the present Fe–(S)Met bond-deprived variants are affected by the motional regime of the protein. In particular, electrostatic adsorption onto a negatively charged SAM surface – which would mimic the phospholipidic inner mitochondrial membrane – facilitates unfolding compared to the solution state, especially at room temperature. This finding has physiological relevance related to the cytochrome c interaction with cardiolipin at the IMM in the early stages of apoptosis. Moreover, while both immobilized variants maintain the His/OH− axial heme iron coordination up to 7 M urea, the same species in solution are subjected to urea-induced replacement of the axial hydroxide ligand by a His ligand. The contributions of the enthalpic and entropic terms to 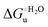 were found to be opposite (H–S compensation), indicating that the unfolding thermodynamics are strongly affected by changes in the hydrogen bonding network in the hydration sphere of the protein.
were found to be opposite (H–S compensation), indicating that the unfolding thermodynamics are strongly affected by changes in the hydrogen bonding network in the hydration sphere of the protein.
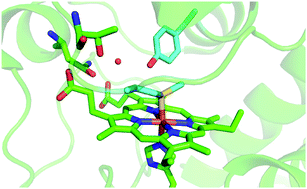
- This article is part of the themed collection: Exploring proteins and their interactions


 Please wait while we load your content...
Please wait while we load your content...