Sub-stoichiometric inhibition of IAPP aggregation: a peptidomimetic approach to anti-amyloid agents†
Abstract
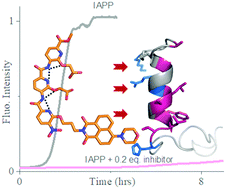
Membrane-catalysed misfolding of islet amyloid polypeptide is associated with the death of β-cells in type II diabetes (T2D). Most active compounds so far reported require high doses for inhibition of membrane bound IAPP fibrillation. Here, we describe a naphthalimide-appended oligopyridylamide-based α-helical mimetic, DM 1, for targeting membrane bound IAPP. DM 1 completely inhibits the aggregation of IAPP at doses of 0.2 equivalents. DM 1 is also effective at similarly low doses for inhibition of seed-catalyzed secondary nucleation. An NMR based study demonstrates that DM 1 modulates IAPP self-assembly by stabilizing and/or perturbing the N-terminus helix conformation. DM 1 at substoichiometric doses rescues rat insulinoma cells from IAPP-mediated cytotoxicity. Most importantly, 0.2 equivalents of DM 1 disaggregate preformed oligomers and fibrils and can reverse cytotoxicity by modulating toxic preformed oligomers and fibrils of IAPP into non-toxic conformations.
- This article is part of the themed collections: Exploring proteins and their interactions and 2021 RSC Chemical Biology HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...