Antibacterial activity of a dual peptide targeting the Escherichia coli sliding clamp and the ribosome†
Abstract
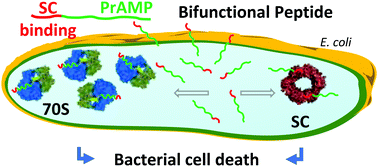
The bacterial processivity factor, or sliding clamp (SC), is a target of choice for new antibacterial drugs development. We have previously developed peptides that target Escherichia coli SC and block its interaction with DNA polymerases in vitro. Here, one such SC binding peptide was fused to a Proline-rich AntiMicrobial Peptide (PrAMP) to allow its internalization into E. coli cells. Co-immunoprecipitation assays with a N-terminally modified bifunctional peptide that still enters the bacteria but fails to interact with the bacterial ribosome, the major target of PrAMPs, demonstrate that it actually interacts with the bacterial SC. Moreover, when compared to SC non-binding controls, this peptide induces a ten-fold higher antibacterial activity against E. coli, showing that the observed antimicrobial activity is linked to SC binding. Finally, an unmodified bifunctional compound significantly increases the survival of Drosophila melanogaster flies challenged by an E. coli infection. Our study demonstrates the potential of PrAMPs to transport antibiotics into the bacterial cytoplasm and validates the development of drugs targeting the bacterial processivity factor of Gram-negative bacteria as a promising new class of antibiotics.
- This article is part of the themed collection: RSC Chemical Biology Transparent Peer Review Collection


 Please wait while we load your content...
Please wait while we load your content...