Tools for functional dissection of site-specific O-GlcNAcylation
Abstract
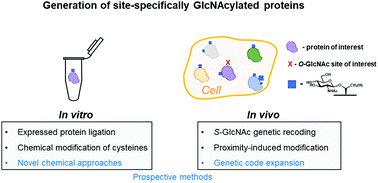
Protein O-GlcNAcylation is an abundant post-translational modification of intracellular proteins with the monosaccharide N-acetylglucosamine covalently tethered to serines and threonines. Modification of proteins with O-GlcNAc is required for metazoan embryo development and maintains cellular homeostasis through effects on transcription, signalling and stress response. While disruption of O-GlcNAc homeostasis can have detrimental impact on cell physiology and cause various diseases, little is known about the functions of individual O-GlcNAc sites. Most of the sites are modified sub-stoichiometrically which is a major challenge to the dissection of O-GlcNAc function. Here, we discuss the application, advantages and limitations of the currently available tools and technologies utilised to dissect the function of O-GlcNAc on individual proteins and sites in vitro and in vivo. Additionally, we provide a perspective on future developments required to decipher the protein- and site-specific roles of this essential sugar modification.
- This article is part of the themed collection: 2021 RSC Chemical Biology HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...