Labelling of DNA and RNA in the cellular environment by means of bioorthogonal cycloaddition chemistry
Abstract
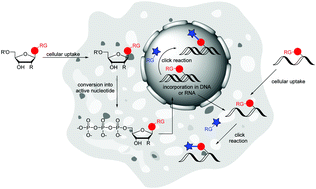
Labelling of nucleic acids as biologically important cellular components is a crucial prerequisite for the visualization and understanding of biological processes. Efficient bioorthogonal chemistry and in particular cycloadditions fullfill the requirements for cellular applications. The broadly applied Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC), however, is limited to labellings in vitro and in fixed cells due to the cytotoxicity of copper salts. Currently, there are three types of copper-free cycloadditions used for nucleic acid labelling in the cellular environment: (i) the ring-strain promoted azide–alkyne cycloaddition (SPAAC), (ii) the “photoclick” 1,3-dipolar cycloadditions, and (iii) the Diels–Alder reactions with inverse electron demand (iEDDA). We review only those building blocks for chemical synthesis on solid phase of DNA and RNA and for enzymatic DNA and RNA preparation, which were applied for labelling of DNA and RNA in situ or in vivo, i.e. in the cellular environment, in fixed or in living cells, by the use of bioorthogonal cycloaddition chemistry. Additionally, we review the current status of orthogonal dual and triple labelling of DNA and RNA in vitro to demonstrate their potential for future applications in situ or in vivo.
- This article is part of the themed collections: Analytical methods in chemical biology, RSC Chemical Biology Editors' Choice and International Open Access Week 2020


 Please wait while we load your content...
Please wait while we load your content...