Dynamic visualization of type II peptidyl carrier protein recognition in pyoluteorin biosynthesis†
Abstract
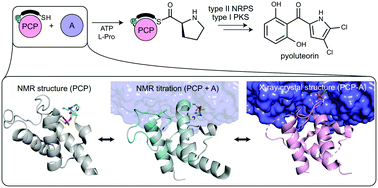
Using a covalent chemical probe and X-ray crystallography coupled to nuclear magnetic resonance data, we elucidated the dynamic molecular basis of protein recognition between the carrier protein and adenylation domain in pyoluteorin biosynthesis. These findings reveal a unique binding mode, which contrasts previously solved carrier protein and partner protein interfaces.
- This article is part of the themed collection: Analytical methods in chemical biology


 Please wait while we load your content...
Please wait while we load your content...