Charge-conversional polyethylenimine-entrapped gold nanoparticles with 131I-labeling for enhanced dual mode SPECT/CT imaging and radiotherapy of tumors†
Abstract
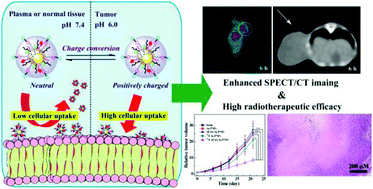
Novel theranostic nanosystems demonstrate great potential to achieve timely diagnosis and effective therapy at the same time. However, due to the relatively low accumulation of theranostic nanosystems at the tumor site, the theranostic efficiency is limited. In this study, a novel theranostic nanosystem with a pH-responsive charge conversion property was constructed to improve the cellular uptake towards cancer cells for enhanced single photon emission computed tomography (SPECT)/computed tomography (CT) dual mode imaging and radiotherapy of tumors. In detail, polyethylenimine (PEI) was utilized as a nanoplatform to link with polyethylene glycol (PEG) monomethyl ether with one end of N-hydroxylsuccinimide (mPEG-NHS), PEG with ends of maleimide and succinimidyl valerate (MAL-PEG-SVA), alkoxyphenyl acylsulfonamide (APAS), 3-(4′-hydroxyphenyl)propionic acid-OSu (HPAO), and fluorescein isothiocyanate (FI), successively. The formed functionalized PEI was then utilized to entrap gold nanoparticles, acetylate the remaining amines of PEI and label with radioactive iodine-131 (131I) to build theranostic nanosystems. The result demonstrated that the theranostic nanosystem has a 3.8 nm Au core and showed excellent colloidal stability. On account of the charge conversion property of APAS, the APAS linked PEI entrapped gold nanoparticles could switch from neutral to positive in a slightly acidic microenvironment, which induced improved cellular uptake. Above all, after 131I labeling, the generated theranostic nanosystem could achieve enhanced SPECT/CT dual mode imaging and radiotherapy of cancer cells in vitro and a xenograft tumor model in vivo. The constructed APAS-linked PEI nanosystem has great potential to be used as a model for SPECT/CT imaging and radiotherapy of various types of cancer.


 Please wait while we load your content...
Please wait while we load your content...