Characterization of regulatory T cell expansion for manufacturing cellular immunotherapies†
Abstract
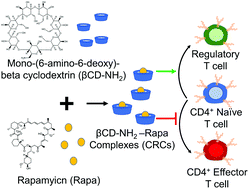
Regulatory T cells (Tregs) are critical mediators of peripheral immune tolerance. Tregs suppress immune activation against self-antigens and are the focus of cell-based therapies for autoimmune diseases. However, Tregs circulate at a very low frequency in blood, limiting the number of cells that can be isolated by leukapheresis. To effectively expand Tregsex vivo for cell therapy, we report the metabolic modulation of T cells using mono-(6-amino-6-deoxy)-β-cyclodextrin (βCD-NH2) encapsulated rapamycin (Rapa). Encapsulating Rapa in β-cyclodextrin increased its aqueous solubility ∼154-fold and maintained bioactivity for at least 30 days. βCD-NH2-Rapa complexes (CRCs) enriched the fraction of CD4+CD25+FoxP3+ mouse T (mT) cells and human T (hT) cells up to 6-fold and up to 2-fold respectively and suppressed the overall expansion of effector T cells by 5-fold in both species. Combining CRCs and transforming growth factor beta-1 (TGF-β1) synergistically promoted the expansion of CD4+CD25+FoxP3+ T cells. CRCs significantly reduced the fraction of pro-inflammatory interferon-gamma (IFN-γ) expressing CD4+ T cells, suppressing this Th1-associated cytokine while enhancing the fraction of IFN-γ− tumor necrosis factor-alpha (TNF-α) expressing CD4+ T cells. We developed a model using kinetic rate equations to describe the influence of the initial fraction of naïve T cells on the enrichment of Tregsin vitro. The model related the differences in the expansion kinetics of mT and hT cells to their susceptibility for immunophenotypic modulation. CRCs may be an effective and potent means for phenotypic modulation of T cells and the enrichment of Tregsin vitro. Our findings contribute to the development of experimental and analytical techniques for manufacturing Treg based immunotherapies.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators 2021


 Please wait while we load your content...
Please wait while we load your content...