A polymeric nanocarrier with a tumor acidity-activatable arginine-rich (R9) peptide for enhanced drug delivery
Abstract
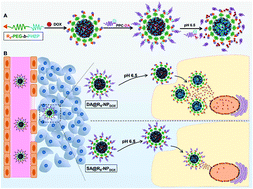
Cell-penetrating peptides (CPPs) have been considered as a powerful tool to improve the intracellular and nuclear delivery efficiency of nanocarriers. However, their clinical application is limited because of their nonspecific targeting function, short half-life, and severe system toxicity. Herein, we have developed a polymeric nanocarrier with a tumor acidity-activatable arginine-rich (R9) peptide for targeted drug delivery. The nanocarrier is fabricated with a R9-conjugated amphiphilic diblock polymer of poly(ethylene glycol) (PEG) and poly(hexyl ethylene phosphate) (PHEP), and then further coated with tumor acidity-activatable polyanionic polyphosphoester through electrostatic interaction in order to block the nonspecific targeting function of the R9 peptide. In the slightly acidic tumor extracellular environment (∼pH 6.5), tumor acidity-activatable polyanionic polyphosphoester would be deshielded from the nanoparticles, resulting in the re-exposure of the R9 peptide to enhance tumor cellular uptake. As a result, intracellular concentration of payload in 4T1 tumor cells significantly increased at pH 6.5. And, we further demonstrate that such a delivery system remarkably promoted the anti-tumor efficiency of chemotherapeutic drugs in tumor-bearing mice, offering great potential for drug delivery and cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...