In vitro cell culture in hollow microfibers with porous structures†
Abstract
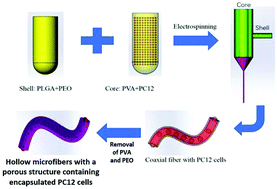
In recent years, there has been a marked increase in tissue engineering research, particularly in the development of electrospun fiber-based substrates as in vitro cell culture platforms. Many scaffolds fabricated via electrospinning have focused on two-dimensional (2D) mats composed of aligned, random or core–shell nanofibers depending on the application and type of extracellular matrix present inside native tissue. In this study, a novel coaxial electrospinning process with a mixture of polylactic-co-glycolic acid (PLGA) and polyethylene oxide (PEO) for sheaths along with polyvinyl alcohol (PVA) for cores was used to produce hollow microfibers. Subsequent removal of PVA and PEO by dissolution resulted in the formation of hollow fibers containing pores inside the shell. The influence of key electrospinning parameters on fiber diameter and pore size was analyzed and optimized via application of design of experiments (DOE). In addition, a regression model was built to show the mathematical relationships and interdependence between process variables and response. Next, the feasibility of this platform for in vitro cell culture was verified by successfully encapsulating and culturing pheochromocytoma 12 (PC12) cells inside the hollow microfibers, where surface perforations could facilitate nutrient and oxygen diffusion and waste removal. After three days of cultivation, it was found that hollow fibers could provide both guidance and support for adherence and proliferation of PC12 cells. This technique based on coaxial cell electrospinning paves the way for development of controllable, tunable and low-cost platforms facilitating guided and constrained culture of cells inside microfibers, thus highlighting its potential as a tool for designing new types of biohybrid materials.


 Please wait while we load your content...
Please wait while we load your content...