Engineering hemoglobin to enable homogenous PEGylation without modifying protein functionality†
Abstract
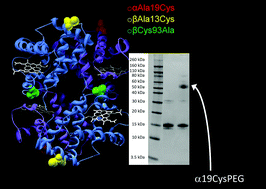
In order to infuse hemoglobin into the vasculature as an oxygen therapeutic or blood substitute, it is necessary to increase the size of the molecule to enhance vascular retention. This aim can be achieved by PEGylation. However, using non-specific conjugation methods creates heterogenous mixtures and alters protein function. Site-specific PEGylation at the naturally reactive thiol on human hemoglobin (βCys93) alters hemoglobin oxygen binding affinity and increases its autooxidation rate. In order to avoid this issue, new reactive thiol residues were therefore engineered at sites distant to the heme group and the α/β dimer/dimer interface. The two mutants were βCys93Ala/αAla19Cys and βCys93Ala/βAla13Cys. Gel electrophoresis, size exclusion chromatography and mass spectrometry revealed efficient PEGylation at both αAla19Cys and βAla13Cys, with over 80% of the thiols PEGylated in the case of αAla19Cys. For both mutants there was no significant effect on the oxygen affinity or the cooperativity of oxygen binding. PEGylation at αAla19Cys had the additional benefit of decreasing the rates of autoxidation and heme release, properties that have been considered contributory factors to the adverse clinical side effects exhibited by previous hemoglobin based oxygen carriers. PEGylation at αAla19Cys may therefore be a useful component of future clinical products.


 Please wait while we load your content...
Please wait while we load your content...