Hemodynamic loads distinctively impact the secretory profile of biomaterial-activated macrophages – implications for in situ vascular tissue engineering†
Abstract
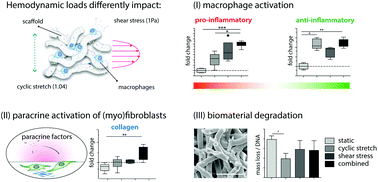
Biomaterials are increasingly used for in situ vascular tissue engineering, wherein resorbable fibrous scaffolds are implanted as temporary carriers to locally initiate vascular regeneration. Upon implantation, macrophages infiltrate and start degrading the scaffold, while simultaneously driving a healing cascade via the secretion of paracrine factors that direct the behavior of tissue-producing cells. This balance between neotissue formation and scaffold degradation must be maintained at all times to ensure graft functionality. However, the grafts are continuously exposed to hemodynamic loads, which can influence macrophage response in a hitherto unknown manner and thereby tilt this delicate balance. Here we aimed to unravel the effects of physiological levels of shear stress and cyclic stretch on biomaterial-activated macrophages, in terms of polarization, scaffold degradation and paracrine signaling to tissue-producing cells (i.e. (myo)fibroblasts). Human THP-1-derived macrophages were seeded in electrospun polycaprolactone bis-urea scaffolds and exposed to shear stress (∼1 Pa), cyclic stretch (∼1.04), or a combination thereof for 8 days. The results showed that macrophage polarization distinctly depended on the specific loading regime applied. In particular, hemodynamic loading decreased macrophage degradative activity, especially in conditions of cyclic stretch. Macrophage activation was enhanced upon exposure to shear stress, as evidenced from the upregulation of both pro- and anti-inflammatory cytokines. Exposure to the supernatant of these dynamically cultured macrophages was found to amplify the expression of tissue formation- and remodeling-related genes in (myo)fibroblasts statically cultured in comparable electrospun scaffolds. These results emphasize the importance of macrophage mechano-responsiveness in biomaterial-driven vascular regeneration.
- This article is part of the themed collection: 29th Annual Conference of the European Society for Biomaterials


 Please wait while we load your content...
Please wait while we load your content...