A spot test for determination of residual TBA levels in 18F-radiotracers for human use using Dragendorff reagent
Abstract
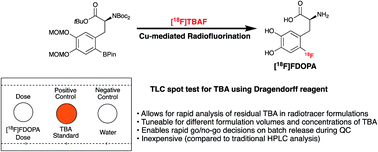
When utilizing [18F]tetrabutylammonium fluoride ([18F]TBAF) in the synthesis of 18F-labeled radiotracers for clinical positron emission tomography (PET) imaging, it is necessary to confirm that residual TBA levels in formulated doses do not exceed established specifications (≤2.6 mg per patient dose). Historically this has been accomplished using HPLC, but this is time consuming for short-lived PET radiotracers and limited by the need for expensive equipment. This motivated us to introduce a TLC spot test for determining residual TBA, and we have developed a new method which employs the Dragendorff reagent. Herein we report details of the TLC method and use it to quantify residual TBA in different formulations of 6-[18F]fluoro-DOPA.


 Please wait while we load your content...
Please wait while we load your content...